Tag: Immunotherapy
-

Targeting Highly Plastic Cancer Cells: A Path to Slower Progression and Better Therapies
Introduction: The Challenge of Cancer Cell Plasticity Cancer is not a uniform mass of identical cells. It is a dynamic ecosystem where a small subpopulation can adapt, change identities, and drive disease progression. Emerging research highlights the role of highly plastic cancer cells—cells that can shift their behavior and characteristics in response to environmental pressures,…
-

When Misdiagnosis Strikes: Autoimmune Encephalitis Misread as Bipolar Disorder
Introduction: A Diagnostic Dilemma In recent years, a relatively new class of autoimmune diseases that attack the brain has prompted psychiatrists and neurologists to reconsider some long-held diagnostic assumptions. Autoimmune encephalitis, though uncommon, often begins with psychiatric symptoms that resemble mood disorders, psychosis, or anxiety. This overlap can lead to misdiagnosis, delayed treatment, and ongoing…
-

Martin Kast’s Lasting Impact on HPV Vaccines and Cancer Education
Pioneering Research on HPV and Cancer Professor W. Martin Kast, the Walter A. Richter Cancer Research Chair at the University of Southern California, has spent more than two decades advancing our understanding of human papillomavirus (HPV) and its link to cancer. His work spans from basic science to translational efforts that bring discoveries from the…
-

Martin Kast’s Impact on HPV Vaccines and Cancer Education
Overview: A Pioneer in HPV Research and Cancer Education Professor W. Martin Kast, the Walter A. Richter Cancer Research Chair at the University of Southern California, has spent more than twenty years advancing our understanding of human papillomavirus (HPV)–related cancers and pioneering new approaches to immunotherapy. His work spans basic science, translational research, and education,…
-
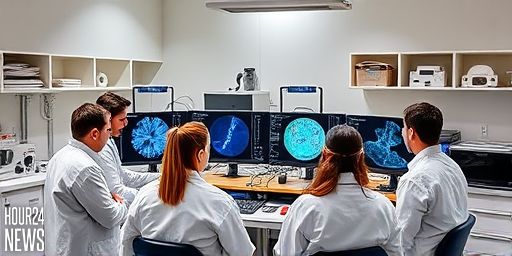
Cryo-EM Maps Autoantibody Hotspots on NMDA Receptors in Autoimmune Encephalitis
New Cryo-EM insights reveal antibody hotspots on NMDA receptors Researchers have leveraged cutting-edge Cryo-Electron Microscopy (Cryo-EM) to pinpoint where autoantibodies most often target NMDA receptors in anti-NMDAR encephalitis. This autoimmune condition, often described in public health circles as “Brain on Fire,” arises when the body’s immune system mistakenly attacks NMDA receptors in the brain, disrupting…
-
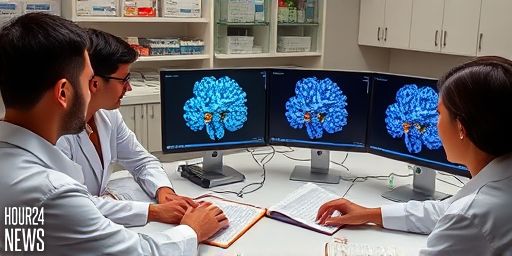
Cryo-EM Maps Spotlight Autoantibody Hotspots on NMDA Receptors for Anti-NMDAR Encephalitis Therapies
New Insights into Anti-NMDAR Encephalitis Autoimmune encephalitis linked to anti-NMDA receptor (NMDAR) antibodies has earned the nickname “Brain on Fire” for its dramatic neuropsychiatric symptoms and rapid progression. A groundbreaking study published in Science Advances leverages cryo-electron microscopy (cryo-EM) to map where autoantibodies most often bind on the NMDAR complex. The findings offer a clearer…
-

A Breakthrough ‘Living Drug’ Gives New Hope to Aggressive Leukemia Patients
Groundbreaking Breakthrough: What is the Living Drug? A new therapeutic approach described as a “living drug” is entering clinical practice for patients with aggressive leukemia. Unlike traditional medicines, this therapy uses living cells engineered to target and destroy cancer cells. The treatment is designed to adapt and respond to the patient’s cancer, offering the potential…
-

New ‘Living Drug’ Brings Hope for Patients with Aggressive Blood Cancer on the NHS
Breakthrough therapy arrives on the NHS A new kind of treatment known as a “living drug” has begun to transform the outlook for patients with aggressive blood cancers. For the first time, an NHS patient has received this innovative therapy, sparking optimism within the medical community and among families affected by the disease. The treatment,…
-

Living Drug Heralds Breakthrough in Aggressive Blood Cancer Treatment on the NHS
New Hope on the NHS: A Living Drug for Aggressive Leukemia A groundbreaking treatment described as a “living drug” has started to change the conversation around aggressive blood cancers in the UK. The first leukemia patient to receive this innovative therapy since its NHS rollout has reported a markedly positive experience, calling the treatment “fantastic”…

