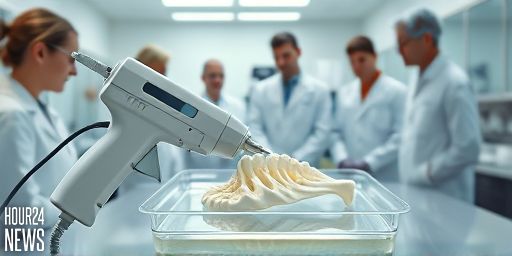
What is the bone-healing gun?
Researchers at Sungkyunkwan University in Korea are pursuing a new approach to bone repair: a device that functions like a handheld 3D printer, but instead of extruding plastic, it lays down a temporary, biodegradable scaffold directly onto a fracture during surgery. The goal is to create patient-specific implants quickly and at lower cost than conventional titanium or metal grafts.
How the technology works
Materials and temperature safety
The team developed a filament that melts at roughly 60 °C, a temperature chosen to protect surrounding tissue. The design uses polycaprolactone (PCL), an FDA-approved thermoplastic, combined with hydroxyapatite to support bone growth. The resulting scaffold bonds to bone and maintains strength during healing while degrading over time.
Biocompatibility and degradation
In lab tests, the composite melts and forms a solid, bone-mimicking framework that gradually breaks down as new bone replaces it. The developers carefully tuned the proportions to balance initial mechanical stability with timely biodegradation, so the scaffold does not stay in the body longer than needed.
Animal studies and early results
After bench tests, the researchers conducted animal trials on rabbits with fractured femurs. Rabbits treated with the bone-healing gun scaffolds recovered faster than those treated with standard bone cement, suggesting the method can speed repair. However, the material’s degradation rate was slower than ideal, which limited full bone restoration in the animal model.
Challenges and future directions
Several hurdles remain before human trials. Degradation rate needs acceleration to allow complete bone replacement in a timely fashion. The team is exploring adding antibiotics to the scaffold to provide infection control during healing. Load-bearing performance is another critical issue; rabbits are lighter than humans, so larger-animal testing will be necessary to assess long-term safety and effectiveness.
Precision and training are also key. The bone-healing gun operates like an advanced glue gun, and surgeons will require specialized training to apply the scaffold consistently and safely during operations. Regulatory pathways, manufacturing scalability, and quality control will also shape the timeline for clinical adoption.
Potential impact
If refined and validated, this approach could cut surgical times, reduce dependence on expensive metal implants, and enable patient-specific scaffolds tailored to fracture geometry. The technology holds promise for faster, less costly recovery and easier customization, potentially transforming how orthopedic fractures are treated in various healthcare settings.