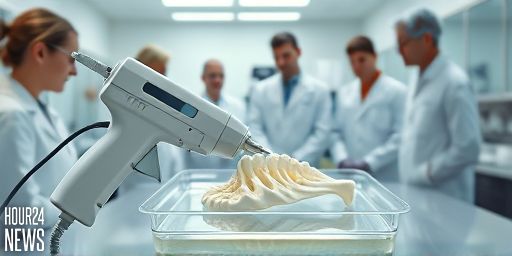
Overview: A new approach to bone repair
Researchers at Sungkyunkwan University in Korea are exploring a speedier, more affordable path to bone stabilization with a device nicknamed the bone-healing gun. Resembling a handheld 3D printer and designed for use during surgery, the tool extrudes biodegradable polymer scaffolds directly onto fractured bone. By forming a custom-fit framework in real time, it aims to support natural bone growth while reducing reliance on expensive metal implants and lengthy manufacturing timelines.
How the bone-healing gun works
The device operates like an advanced hot-melt applicator. It melts biocompatible polymer pellets at a safe, tissue-friendly temperature of about 60 °C, well below temperatures that could harm surrounding tissue. The melted material is dispensed directly onto the fracture site, where it quickly hardens into a scaffold that anchors the bone and guides new bone formation. The end result is a patient-specific implant created in the operating room, potentially lowering costs and shortening recovery times compared with traditional titanium-based solutions.
Materials chosen for strength and safety
The research team settled on a blend of polycaprolactone (PCL), an FDA-approved thermoplastic known for safe biodegradation in months, and hydroxyapatite, a mineral that supports bone growth. After testing various mixtures, they identified a composition that melts at a mild 60 °C, bonds securely to bone, maintains strength during healing, and gradually degrades as natural tissue replaces it. This balance addresses three critical challenges: keeping the temperature safe for living tissue, providing bone-like structural support, and ensuring controlled biodegradation.
Early results from animal trials
Initial testing involved rabbits with femur fractures. Animals treated with the bone-healing scaffold recovered more quickly than those receiving standard bone cement, a common commercial option. While promising, the material’s slower overall degradation prevented complete remodeling of the fracture site, highlighting the need for further refinement before human trials. The findings suggest that, with optimization, the device could shorten healing timelines while avoiding some of the drawbacks of conventional implants.
What researchers hope to optimize next
To move toward clinical use, the team plans to accelerate degradation rates and explore incorporating antibiotics into the scaffold. This could enable the implant to release infection-fighting drugs gradually as healing proceeds. Another key hurdle is load-bearing capability; rabbit models are lighter than humans, so the device must be tested in larger animals to assess long-term safety and mechanical performance under human-like stresses. Additionally, the technique requires precise operation, so surgeons will need targeted training to ensure consistent, safe deployment in real procedures.
Implications for the future of orthopedic surgery
If validated through larger-animal and eventual human studies, the bone-healing gun could offer a faster, more affordable route to patient-specific implants. By enabling on-the-spot customization, the approach has the potential to reduce implant costs, shorten surgery times, and tailor treatment to individual anatomy. While still in early stages, this line of work highlights a broader shift toward regenerative, bioresorbable solutions that work in harmony with the body’s natural healing processes.