Introduction: A Small, Smart Leap in Actuation
Researchers are redefining how soft robotic systems move at the microscale. The development of 3D-printed, low-voltage-driven ciliary hydrogel microactuators marks a significant advance in soft robotics and microfluidics. Unlike millimetre-scale hydrogel devices that relied on interfacial pH or osmotic gradients, these micrometre-scale actuators operate through internal ion migration enabled by nanometre-scale pores. The result is precise, programmable motion with minimal energy input, opening doors to compact, efficient devices that can operate in delicate environments without external catalysts.
How these microactuators work: physics in a tiny package
The core principle centers on ion migration within a hydrogel scaffold that contains nanopores smaller than a thousandth of a millimeter. When a low electrical voltage is applied, charged ions migrate through the pores, creating localized osmotic and electrochemical gradients that drive bending, curling, or protrusive motion of cilia-like structures. Since the hydrogel’s porosity is engineered at the nanoscale, ion transport can be fast and directional while dissipating heat—crucial for maintaining the material’s integrity at micro scales. This mechanism contrasts with older approaches that used bulk chemical gradients, enabling faster response, repeatable actuation cycles, and improved compatibility with embedded microelectronics.
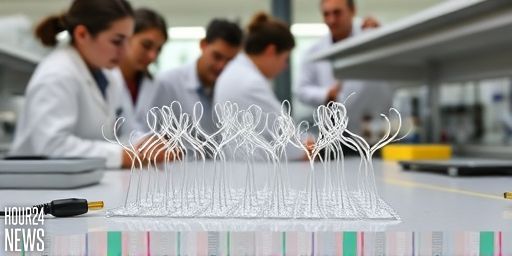
3D printing: tailoring geometry for precise ciliary motion
3D printing enables rapid prototyping of hydrogel microactuators with controlled topology. Designers can tune filament orientation, crosslink density, and pore architecture to modulate stiffness, bending radius, and actuation amplitude. In practice, this means arrays of microscopic cilia can be created to produce coordinated motion or selective grabbing, releasing, or transporting of micro-objects. The printing process also supports the integration of conductive components and insulating layers, allowing the devices to be wired for low-voltage operation without compromising flexibility.
Performance highlights: low voltage, high compliance
Key performance metrics for these actuators include operating voltages in the low-voltage range, high strain response, and robust cycling. The micrometre-scale architecture reduces the energy required to achieve a given displacement, while the nanoscale pores ensure rapid ion exchange during each cycle. This combination yields actuation with high compliance, essential for delicate manipulation tasks in microfluidic channels, biosensors, and soft robotic grippers. Importantly, the architecture minimizes electrical heating and allows operation in aqueous environments, which is ideal for lab-on-a-chip and biomedical applications.
Applications: where tiny, smart actuators shine
The potential applications are broad. In microfluidics, arrays of ciliary hydrogel actuators can drive fluid mixing, transport, or sorting without bulky external pumps. In biocompatible robotics, soft grippers can handle delicate tissues or cells with gentle, programmable motions. Environmental sensing can leverage responsive hydrogel fibers that bend toward stimuli, acting as micro-scale valves or mixers in tiny analytical devices. Additionally, the compatibility with standard 3D printing workflows reduces manufacturing barriers, enabling researchers and engineers to customize devices for specific tasks quickly.
Advantages over traditional microactuators
Compared with conventional actuators, these hydrogel systems benefit from:
– Low-voltage operation that preserves surrounding components and minimizes power demands.
– Internal ion migration driven by nanoscale pores for rapid, reversible actuation.
– Additive manufacturing compatibility for rapid iteration and customization.
– Intrinsic softness that reduces wear and tear on delicate microenvironments.
– Potential for autonomous sensing–actuation loops when integrated with simple electronics.
Future directions: from lab curiosity to practical tools
To transition from proof-of-concept to widespread use, ongoing work focuses on improving long-term durability, environmental stability, and the integration of sensing modalities with actuation. Researchers are also exploring multi-material printing to create composite hydrogel systems that combine stiffness gradients with embedded electronics. In time, 3D-printed, low-voltage-driven ciliary hydrogel microactuators could become foundational elements in micro-robotic systems, lab-on-a-chip platforms, and responsive biosensing devices.
Conclusion
The advent of micrometre-scale, 3D-printed hydrogel microactuators driven by internal ion migration represents a meaningful stride toward compact, energy-efficient soft robots. By leveraging nano-porous hydrogel architectures and precise 3D printing, researchers are crafting ciliary actuators that perform complex tasks with minimal electrical input, paving the way for versatile micro-robotics and microfluidic technologies that fit onto chips and in tiny devices.